Abstract
Introduction: Acalabrutinib (Acala) and ibrutinib (Ibr), as single agents, are both highly effective Bruton tyrosine kinase inhibitors approved for the treatment of relapsed/refractory mantle cell lymphoma and chronic lymphocytic leukemia. Both are given as continuous oral treatments until disease progression or unacceptable toxicity. In the absence of a head-to-head clinical study in this setting, this analysis aims to compare the efficacy and safety of Acala versus Ibr for the treatment of relapsed/refractory mantle cell lymphoma (MCL) using matching-adjusted indirect comparison (MAIC). This MAIC slightly differs from the published analysis by Telford et al (Clin Ther. 2019;41:2357-79) by using long-term follow-up data for efficacy outcomes and assessing whether the safety profile of Acala is sustained over a longer term when compared with Ibr.
Methods: Individual patient data for Acala from LY-004 (38 months median follow-up) (Wang et al. ASCO 2021) were weighted to match the aggregate baseline characteristics of the Ibr monotherapy arm from a pooled analysis with 41.4 months median follow-up (Rule et al. Hematologica. 2019.04:e212). These baseline characteristics, Eastern Cooperative Oncology Group performance status, simplified MCL International Prognostic Index score, lactate dehydrogenase, prior lines of therapy, bulky disease, and blastoid histology, are potential prognostic variables (PVs). Pseudo-individual patient data were generated from the digitized Kaplan-Meier curves published in the aforementioned comparator trials. As the LY-004 trial is a single-arm trial, an unanchored MAIC was conducted to adjust for these PVs. The PVs selected were based on literature, clinical judgment, and demonstrated statistically significant association with progression-free survival (PFS) in univariate and multivariate regression analysis (Ahn et al. J Clin Oncol. 2020;39:576-85; Eichorst and Hallek. Hematol Am Soc Hematol Educ Prog. 2016;1:149-55). After matching, a weighted Cox proportional-hazards model was used to analyze PFS and overall survival (OS) while a weighted logistic regression model was used for comparative safety analysis (grade ≥3 adverse events [AEs]). Two-sided P<0.05 was considered statistically significant.
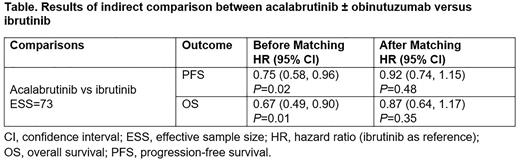
Results: In the Acala vs Ibr comparison before matching, PFS and OS favored Acala and were statistically significant. After matching, PFS and OS numerically favored Acala but the difference was not significant (Table). In the Telford et al study, significant differences were observed in grade 3/4 atrial fibrillation (afib) and thrombocytopenia. Similarly, in the current analysis, rates of afib and thrombocytopenia were significantly lower with Acala vs Ibr. In addition, rates of grade 3/4 AEs were numerically lower with Acala than Ibr for neutropenia, pneumonia, and hypertension.
Conclusions: Based on these MAIC results, in addition to a similar efficacy benefit vs Ibr, Acala was associated with a consistent safety profile over the long term. A limitation of this MAIC is not including all potential PVs as a trade-off to conserve the effective sample size. However, the use of longer follow-up data improved the robustness of the indirect comparisons of safety and efficacy.
Disclosures
Gaitonde:AstraZeneca: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Cai:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. de Miranda:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Roos:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Rule:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Wang:Oncology Specialty Group: Honoraria; OncLive: Honoraria; Meeting Minds Experts: Honoraria; Dava Oncology: Honoraria; MJH Life Sciences: Honoraria; Acerta Pharma: Honoraria, Research Funding; Oncternal: Consultancy, Research Funding; AbbVie: Consultancy; Celgene: Research Funding; VelosBio: Consultancy, Research Funding; Medscape: Honoraria; Moffit Cancer Center: Honoraria; Juno Therapeutics: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Molecular Templates: Research Funding; Practice Point Communications (PPC): Honoraria; Studio ER Congressi: Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; IDEOlogy Health: Honoraria; LLC TS Oncology: Honoraria; Vinverx: Research Funding; Deciphera: Consultancy; Loxo Oncology: Research Funding; InnoCare: Consultancy, Research Funding; Genmab: Research Funding; Genentech: Consultancy, Research Funding; Milken Institute: Consultancy; Merck: Honoraria; Physicians Education Resources (PER): Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; Leukemia & Lymphoma Society: Consultancy, Honoraria; Pepromene Bio: Consultancy; Lilly: Consultancy, Research Funding; Kite Pharma: Consultancy, Honoraria, Research Funding; BioInvent: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal